Microorganisms, encompassing bacteria, fungi, viruses, and beyond, are tiny life forms pervading our planet. They dwell in soil, water, air, and even within our own bodies. Despite frequently escaping our notice in our day-to-day routines, rigorous scientific research has overwhelmingly underscored their paramount role within Earth’s intricate ecosystems. Indeed, microorganisms can be rightfully regarded as elemental to the sustenance and flourishing of all the Earth’s plant and animal life.
Examined from both human and environmental perspectives, microorganisms fulfill indispensable roles. They undertake vital ecological duties within their environment, including the decomposition of organic matter, the cycling of nutrients, and the enrichment of soil fertility. Besides, microorganisms have the capability to generate antibiotics and other advantageous compounds. These are extensively used in medical and pharmaceutical research, fortifying our defenses against pathogens and an array of diseases. Nevertheless, it’s crucial to acknowledge that certain microorganisms are capable of causing diseases that jeopardize human health and the well-being of various life forms. Thus, scientists also dedicate in research to unravel the disease-causing mechanisms and infection routes, among other vital aspects. This profound comprehension of the pathogenic mechanisms of microorganisms empowers the development of more effective strategies for prevention, diagnosis, and treatment, ultimately safeguarding the health of both fauna and flora.
Among plant-associated bacteria, agrobacteria hold a distinctive position. They are prevalent inhabitants of environments like soil and the root systems of plants, fostering intimate associations with a diverse plant species. Agrobacteria, classified as a type of plant pathogen, have the capacity to infect plant wound sites, inciting crown gall or hairy root diseases. Furthermore, their interkingdom horizontal gene transfer ability allows them to incorporate foreign DNA fragments into plant cells, consequently provoking genetic modifications. This unique attribute renders agrobacteria an indispensable asset in genetic engineering and gene functionality exploration.
Many pathogenic bacteria have evolved specialized protein secretion systems that enhance their pathogenicity or competitive advantage. Agrobacteria, in this case, employ the type IV secretion system (T4SS) to transport the transfer DNA (T-DNA) into plant cells, while also utilizing the type VI secretion system (T6SS) to deliver effectors to kill or inhibit the growth of their competitors. Therefore, the research into the functionality, regulatory mechanisms, and evolutionary processes of agrobacterial secretion systems not only contributes to improving plant genetic transformation efficiency but also propels advancements in agriculture. Furthermore, it provides crucial insights for the development of relevant drugs aimed at controlling pathogen infections in plants and animals.

Agrobacterium has diverse weapons and deploys different adaptation strategies when facing various environments and contact targets. It collaborates with allied forces and activates the type VI secretion system (T6SS) to defend the enemies. When encountering suitable plant hosts, it rapidly produces virulence proteins and collaboratively transports T-DNA into plant cells through the type IV secretion system (T4SS), inducing tumor formation in plants for its residence and reproduction.
(This figure and caption are sourced from the popular science column from Academia Sinica newsletter (November 2020), titled “Genetic Engineer: Agrobacterium’s Competition and Pathogenic Mechanisms“. Figure was drawn by Dr. Yu Wu, a Ph.D. candidate in the Molecular and Biological Agricultural Science Program in Taiwan International Graduate Program.)
The research team led by Dr. Erh-Min Lai at the Institute of Plant and Microbial Biology, Academia Sinica, has been conducting numerous studies on the role of the T6SS in agrobacteria. Previous studies have revealed that agrobacteria secrete DNase toxin effectors called Tde (Type VI DNase effectors). Agrobacteria use these effectors to attack co-existing competitors by injecting Tde toxins into competitor cells, thereby gaining a competitive advantage for agrobacteria survival in their ecological niche or inside the plant host. Over the years, multiple research groups have also uncovered various T6SS effector proteins, each with a diverse set of functions spanning different cellular locations, including the cell membrane, periplasm, and cytoplasm. However, the entry process of these effector proteins into target cells remain unclear.
Recently, Dr. Erh-Min Lai’s laboratory utilized the Tde1 from the soil-borne phytopathogen Agrobacterium strain C58 as the research subject on uncovering the mechanism behind effector proteins entering target cells. The results of this research emphasize the critical function of the N-terminal glycine zipper motifs of Tde1 in facilitating the delivery of effector proteins into target cells.
The findings revealed that the N-terminus of Tde1 containing a single transmembrane domain overlapping with repeated glycine zipper motifs can cause membrane permeabilization in a G39xxxG43-dependent manner. Through various genetic, biochemical, and microscopy analyses, the laboratory found that the N-terminus of Tde1 is necessary and sufficient for secretion and translocation into target cells. The amino acid substitution of G39xxxG43 motif retains the Tde1 DNase activity and its interaction with Tap1 for secretion but abolishes its delivery into target cells., suggesting that the G39xxxG43 motif of Tde1 is critical for target cell delivery and bacterial killing in DNase-dependent manner. Collectively, the laboratory discovered that Tde1 DNase effector employs glycine zipper for translocation into target cells, representing a novel role of glycine zipper in effector delivery instead of toxicity.
Considering the widespread presence of glycine zipper motifs in bacterial effectors besides pore-forming toxins, the laboratory proposed that this glycine zipper-mediated delivery could be a common strategy deployed by many bacterial toxins for translocation across target cell membranes. This finding provides a theoretical basis of using the non-toxic effector module for the delivery of proteins of interests such as genetic modifiers into target cells. This strategy also offers an advantage over transforming foreign DNA for expressing a protein of interest from creating undesired genome manipulation.
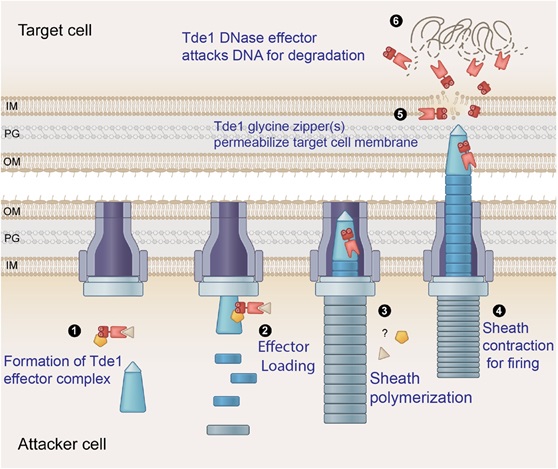
(The figure is adopted from Ali et al., EMBO reports. (2023) 24:e56849.)
Step 1: Effector proteins Tde1 form a complex with its immunity protein Tdi1 and adaptor protein Tap1 in the attacker cell.
Step 2: Tap1-Tde1-Tdi1 complex binds to the VgrG protein, and is loaded onto the membrane-associated baseplate.
Step 3: The Tde1-loaded baseplate begins to form a tubular telescopic device.
Step 4: The sheath contracts and ejects out Tde1 into target cells.
Step 5: The N-terminus of Tde1 glycine zipper(s) permeabilize target cell membrane.
Step 6: Upon entering the target cell, Tde1 attacks DNA for degradation.
Reference:
Jemal Ali, Manda Yu, Li-Kang Sung, Yee-Wai Cheung, Erh-Min Lai*
A glycine zipper motif is required for the translocation of a T6SS toxic effector into target cells. EMBO reports. (2023) 24:e56849
https://doi.org/10.15252/embr.202356849
