
Have you ever wondered how the surface of green leaves forms as you admire their beauty? In fact, the surface of these green leaves is covered by a tissue called the leaf epidermis.
The leaf epidermis acts as a protective shield for leaves, serving as the first line of defense between plants and the external environment. It not only safeguards leaves from damage but also regulates gas exchange and water loss between plants and the surroundings. Additionally, the leaf epidermis serves a defensive function, fending off threats such as drought, ultraviolet radiation, and pathogen invasion. Therefore, studying the development of leaf epidermis not only enhances our understanding of plant growth but also significantly contributes to the resilience of crops in the face of climate change-induced extreme weather conditions.
The leaf epidermis is composed of various types of cells, including stomata, trichomes, and pavement cells.
Stomata, consisting of a pair of guard cells, regulate the exchange of gases (such as carbon dioxide and oxygen) and water transpiration. The opening and closing of stomata are influenced by both internal and external environmental factors, such as light intensity, humidity, and carbon dioxide concentration.
Trichomes serve as a defense mechanism against insect bites, with some emitting volatile chemicals to repel insects.
Pavement cells, along with their cuticle layers on the top, prevent water loss and require highly coordinated and specialized development to form a cohesive tissue.
In the past, scientists primarily used genetic dissection to study their formation. However, emerging quantitative measurement methods allow for the monitoring of cellular and tissue dynamics, enabling further exploration of cell state transitions and other processes involved in leaf epidermis development.
Dr. Chin-Min Kimmy Ho and the research team from the Institute of Plant and Microbial Biology at the Academia Sinica have used the formation of epidermal cell types in Arabidopsis as an example to introduce various quantification tools and methods.
These quantification methods provide quantitative data, such as shape parameters, cell numbers, sizes, and morphologies, which can be used to compare differences between different cell states or track changes in cells during development. By tracking the transitions in cell states, we can begin to understand the decisions made by cells during the developmental process, including cell differentiation and determination of cell fate.
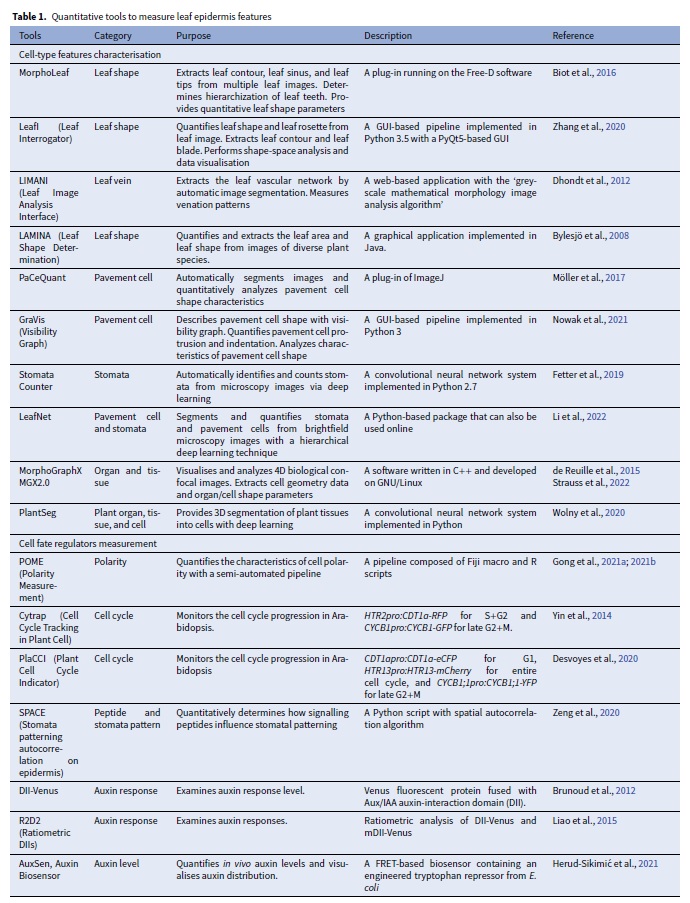
Taking leaf shape measurement as an example, Arabidopsis forms elliptical true leaves with serrated edges. Classical parameters such as leaf area, length (height), width, and circularity are used to describe the shape of the leaves. The aspect ratio determines whether the leaf is more circular or elliptical, while leaf circularity reflects the degree of serration along the leaf margin. In 2008, the program LAMINA (Leaf shApe deterMINAtion) was developed to extract leaf measurement data from scanned leaf images, providing accurate results for both single and compound leaves. LAMINA can be employed to determine the number of leaf serrations along the leaf margin and the boundary coordinates, describing the degree of asymmetry in leaf shape. Additionally, in Arabidopsis, there is no clear boundary between the leaf blade and the petiole, which may hinder the quantification of overall leaf area (including the petiole). In such cases, the program MorphoLeaf can be used to manually remove the petiole, improving the accuracy of leaf shape quantification.
In the measurement of pavement cells, various parameters have been used to describe their characteristics, including the aspect ratio, the number of lobes and indentations, circularity, solidity, and convexity. Programs such as PaCeQuant, LobeFinder, and LOCO-EFA utilize a boundary-based approach to detect the lobes in pavement cells. Among these, PaCeQuant provides 27 pavement cell-shape features and can automatically detect cell boundaries from confocal images, as well as accept segmented cell fragments to gather data.
Research on leaf epidermal development holds significant importance in both the agricultural and biological fields.
In agriculture, the development of leaf epidermis is closely related to a plant’s ability to withstand stress and its overall yield. For instance, reducing the number of stomata in rice or enhancing the speed of stomatal closure can increase the crop’s drought tolerance and water use efficiency. These strategies can be beneficial for plants to better survive and grow under extreme climate-induced drought conditions in the future. Furthermore, the leaf epidermis also plays a crucial role in determining a plant’s defense mechanisms against pathogens and pests. By gaining deeper insights into the molecular mechanisms and physiological processes involved in leaf epidermal development, we can enhance our understanding of the fundamental aspects of plant growth and development. This expertise, in turn, provides valuable information for future agricultural production and fundamental scientific research.
Plant growth is a dynamic process that leads to a diverse population. Quantitative biology provides methods and tools to describe this diversity. Scientists are actively exploring various quantitative approaches to delve deeper into the mysteries of leaf epidermal development, enriching our comprehensive understanding of leaf epidermal cell differentiation. Moreover, the introduction of emerging technologies assists scientists in studying the dynamics of cells or tissues, further enhancing our knowledge of the leaf epidermal development process. In the long term, there will be a continued exploration of the interactions and communication mechanisms among different cell types during leaf epidermal development. This knowledge will be applied in agricultural production and fundamental scientific research, driving the cultivation of crop varieties with enhanced resilience.
Reference:
Chi Kuan, Shao-Li Yang and Chin-Min Kimmy Ho*(2022)
Using quantitative methods to understand leaf epidermal development. Quantitative Plant Biology.
https://dx.doi.org/10.1017/qpb.2022.25
